Abstract
Background: Large epidemiologic studies have identified important clinical risk factors for AML development; however their prevalence and association with AML cytogenetic subgroups and outcome after therapy have not been studied prospectively. To study clinical exposures and systematically capture risk factors, we incorporated a novel epidemiology study using the ' ALESE' Questionnaire (AQ) in the large phase III Intergroup E2906 randomized trial of intensive therapy in older adults with newly diagnosed AML. This is the first report of ALESE.
Methods: E2906 studied therapy in patients >/= 60 years of age with AML, and the primary objective demonstrated superiority of cytarabine and daunorubicin over clofarabine, and has previously been presented (ASH 2015, abs. #217). All E2906 patients had a central review of karyotype for confirmation and adjudication, and cytogenetic risk was assigned into 4 groups [Core Binding Factor Leukemia (CBL), Cytogenetically Normal AML (CN-AML), Intermediate Abnormal, and Adverse] according to the Grimwade Classification. E2906 began in January 2011, and all patients registered to E2906 after May 2013 were eligible to complete the AQ, within 7 days of randomization when possible. The AQ comprises 100 questions on clinical and occupational exposures; personal and family medical history including medication exposures; race and socioeconomic status (SES); and lifestyle factors including smoking history. AQ is self-administered and takes 20-30 minutes to complete. Using case-case comparisons, associations of exposures with each specific cytogenetic risk group (i.e. CBL (n=14) vs. other, CN-AML (n=127) vs. other, intermediate abnormal (n=77) vs. other, and adverse (n=90) vs. other) were evaluated using logistic regression models. Only unadjusted models were utilized for the CBL vs. other analysis due to the small sample size of the CBL group, while for all other analyses multivariable models were utilized and adjusted for variables that differed between cytogenetic risk groups at p<0.05 in unadjusted analysis. Odds Ratios (ORs) and 95% confidence intervals (CIs) were estimated. A nominal p<0.05 was used to declare statistical significance in this exploratory study.
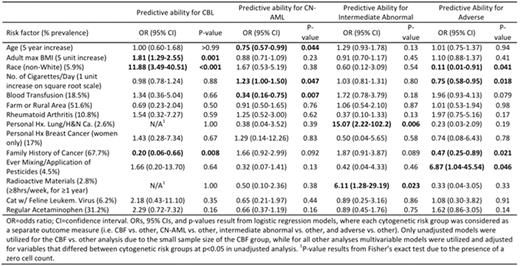
Results: The AQ return rate among eligible patients was 69% (n=363/527), and this report represents the first analysis, which includes 308 patients with confirmed central cytogenetics review and risk group assignment. The median age in this cohort was 67 years (range 60-84), and the median BMI at AML diagnosis was 27.4 kg/m2 (range 16.1-48.8) with 30.9% BMI ≥ 30. The prevalence of dichotomous exposures is reported in the Table. Using multivariable logistic regression models, we identified several novel associations of epidemiologic exposures with unique cytogenetic risk groups (Table). The CBL group was positively associated with maximum adult BMI and non-white race and was inversely associated with a family history (FHx) of cancer. CN-AML was positively associated with the number of cigarettes/day and was inversely associated with age and history of blood transfusion. The intermediate abnormal risk group was positively associated with a personal history of lung or head and neck cancer, and exposure to radioactive materials. Finally the adverse risk group was positively associated with mixing/application of pesticides and inversely associated with non-white race, number of cigarettes/day and FHx of cancer.
Conclusion: The ALESE study documents the ability to collect epidemiology data within a clinical trial, and establishes the prevalence of important clinical epidemiologic exposures associated with leukemia development in older AML patients in a randomized study population. We have identified several novel associations of epidemiologic and clinical exposures with specific cytogenetic risk groups, and these factors were largely unique to only one risk group. While requiring replication, these initial observations could be clinically relevant, lend novel insights into risk factors for development of cytogenetic lesions, and support the importance of developing novel high impact epidemiology studies embedded in clinical trials. The ALESE study will directly inform future studies of leukemia risk including assessment of etiologic heterogeneity of AML subtypes, possible mechanisms of leukemogenesis, and ultimately the development of leukemia prevention strategies.
Uy: Novartis: Consultancy, Other: Travel Suppport; Boehringer Ingelheim: Consultancy; GlycoMimetics: Consultancy. Erba: Celgene: Consultancy, Other: Chair, Scientific Steering Committee , Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Incyte: all research support paid to University of Alabama, Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Amgen: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Daiichi Sankyo: Consultancy, Other: all research support paid to University of Alabama, Research Funding; ImmunoGen: Consultancy, Other: all research support paid to University of Alabama, Research Funding; MacroGen: Consultancy; Ono: Consultancy; Pfizer: Consultancy; Seattle Genetics: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Sunesis: Consultancy; Millennium/Takeda: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Agios: Other: all research support paid to University of Alabama, Research Funding; Juno: Other: all research support paid to University of Alabama, Research Funding; Astellas: Other: all research support paid to University of Alabama, Research Funding; Celator: Other: all research support paid to University of Alabama, Research Funding; Janssen: Other: all research support paid to University of Alabama, Research Funding; Glycomimetics: Other: Chair, Data and Safety Monitoring Committee. Powell: Rafael Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cerhan: Janssen: Other: Multiple Myeloma Registry Steering ; Janssen: Other: Scientific Advisory Board (REMICADELYM4001).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal